Research

The brain contains billions of nerve cells, or neurons, which receive and integrate signals from the environment and govern the body’s responses. Neuronal activity is made possible by synapses, contacts between neurons or between a neuron and a target cell. Neurotransmitter molecules are released from the presynaptic membrane and activate receptors on the postsynaptic membrane, thus establishing neuronal communication. As such, synapses are fundamental units of neural circuitry and enable complex behaviors.
Research in our lab has focused on mechanisms of synapse formation, neurotransmission, and synaptic plasticity. Our goal is to contribute to a better understanding of these processes and development of potential therapeutic strategies for psychiatric disorders such as schizophrenia, autism, and depression and neurological disorders such as neuromuscular disorders and epilepsy.
The neuromuscular junction (NMJ) formation and disorders

Our daily activities – get out of the bed, walk, eat, drink, and sit – requires the proper function of the NMJ, a peripheral synapse between motoneurons and muscle fibers that is critical for accurate and rapid control of muscle contraction. This classic chemical synapse has served as an informative model of synapse structure and function. Although many synaptic components have been identified, how they are assembled to form the NMJ remains unclear. The NMJ formation requires agrin, a trophic factor from motoneurons and MuSK, a transmembrane tyrosine kinase that can be activated by agrin; however, agrin does not interact with MuSK. We identified LRP4 as a receptor for agrin and revealed mechanisms how signals are transduced from agrin to MuSK by solving a crystal structure of an agrin-LRP4 complex. We have discovered antibodies against LRP4 and agrin in patients with myasthenia gravis and demonstrated that they are causal to myasthenia gravis. These antibodies are used as novel biomarkers for diagnosis of myasthenia gravis. We demonstrated that rapsyn, a classic scaffold protein, undergoes liquid-liquid phase separation to form membraneless condensates for AChR clustering and NMJ formation. In addition, rapsyn possesses an E3 ligase activity that is required for NMJ formation. We have revealed novel pathways that are critical for the development, maintenance and regeneration of the NMJ.
Neuregulin 1 (NRG1) and ErbB4 signaling in excitation-inhibition balance
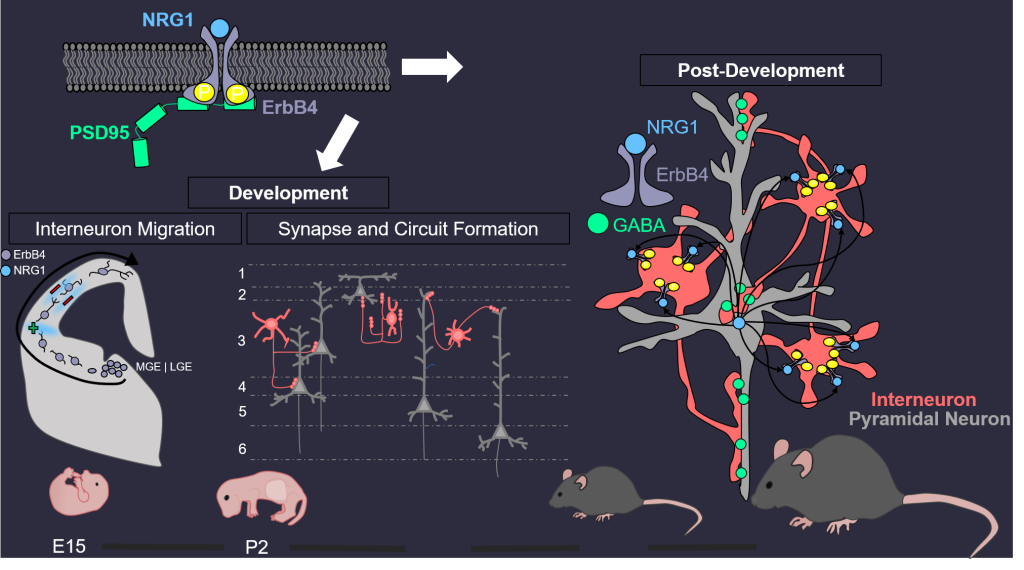
There are two main types of neurons: excitatory (also called projection or pyramidal) neurons that use glutamate as neurotransmitter and inhibitory interneurons (INs) that release GABA, Comprising 15%–20% of total neurons in the brain, INs control the excitability of projection neurons. Via feedforward and feedback inhibition, interneurons increase the computational power of cortical networks and synchronize both local and distant cortical circuits that are key to oscillatory activity. Disruptions of GABA signaling have been implicated in brain disorders including autism, depression, intellectual disability, Rett syndrome, and schizophrenia. We have demonstrated that NRG1 and its receptor ErbB4 are critical to GABA activity in the brain. NRG1 is produced by pyramidal neurons in an activity-dependent manner. It binds to ErbB4 in INs to promote GABA release, and consequently suppresses the activity of pyramidal neurons. This homeostatic pathway is critical to many brain functions including working memory and fear. Translationally, both NRG1 and ErbB4 are risk genes of major depression disorders and schizophrenia. Our research has provided insight into pathophysiological mechanisms of devastating brain disorders.
Pathological mechanisms of brain disorders
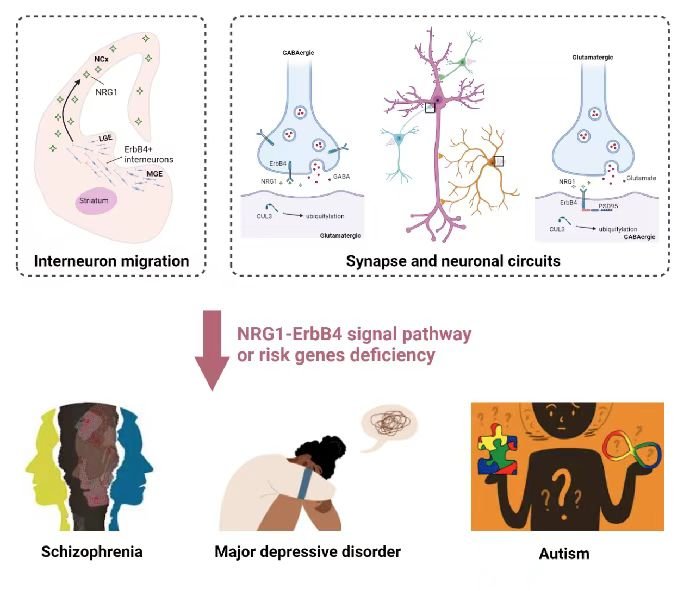
Many psychiatric disorders lack clear pathological hallmarks and thus are poorly understood in terms of pathophysiological mechanisms. With the advance in powerful genetic analysis, risk genes have been identified for various brain disorders. By studying mouse models of deficiency and/or de novo mutations of Erbin, TMEM108, NRG3, Caspr3, and Cullin 3 (images below, left wildtype, right Cullin 3 deficient mice), our studies reveal their physiological functions in neural development and neurotransmission and provide insight into pathophysiological mechanisms of schizophrenia, major depression and autism.